Abstract
Background and rationale Women and girls with one or two abnormal alleles of clotting factor genes can experience hemophilia symptoms, but a lack of data exists on the treatment of women with hemophilia. We used the ATHNdataset to identify female patients who received octocog alfa (BAY 81-8973/Kovaltry®) or damoctocog alfa pegol (BAY 94-9027/Jivi®) between January 1, 2010 and April 30, 2022. Baseline demographics, treatment history and medical history of patients aged ≥12 years with hemophilia A for damoctocog alfa pegol and all ages for octocog alfa were collected. The HIPAA-compliant, de-identified database, ATHNdataset, is a national database sponsored by the American Thrombosis and Hemostasis Network. This repository contains patient data, including of those with hemophilia A, gathered from ATHN-affiliated hemophilia treatment centers across the United States.
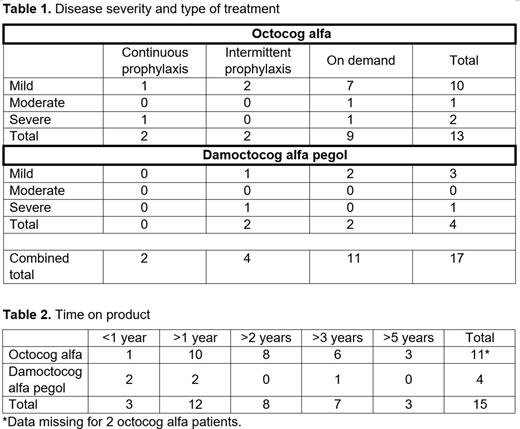
Results: A total of 354 and 205 patients receiving octocog alfa and damoctocog alfa pegol, respectively, were in the ATHNdataset at the time of data cutoff. Of these, 13 patients receiving octocog alfa and four receiving damoctocog alfa pegol were female. Of the patients receiving octocog alfa, there was one pediatric patient (<12 years), two adolescent patients (12-18 years), and ten adult patients (>20 years) of whom seven were older than 30 years. For damoctocog alfa pegol, there were two adolescent patients (12-18 years) and two adults (>50 years). These patients were treated as listed in Table 1. For the octocog alfa patients, out of 11 patients, only one was treated with octocog alfa for <1 year, and six patients were treated for >3 years. Data regarding length of treatment were missing for two patients. Two patients were treated with damoctocog alfa pegol for <1 year, and two for >1 year with one of them for >3 years (Table 2). Of the octocog alfa patients, there was one patient who had four bleeds; all bleeds were traumatic, including three joint bleeds. One patient receiving damoctocog alfa pegol had one traumatic bleed with no joint bleeds. All other patients had zero bleeds. Eight patients on octocog alfa with known data were previously treated with another product. Five of these eight patients were on a previous standard half-life product. Three of the eight had bleeding episodes, including spontaneous and joint bleeds, on their previous product; one patient with five bleeds had two traumatic bleeds and three joint bleeds. None of these patients had bleeds while using octocog alfa. All these patients were classified as having mild hemophilia; there was also one patient with moderate and two with severe hemophilia. Two patients on damoctocog alfa pegol with known data were previously treated with another product, one episodically with desmopressin and the other prophylactically with an extended half-life product. Neither had any bleed data listed in the dataset. Previous product usage of the patient with one traumatic bleed on damoctocog alfa pegol was unknown. Three patients receiving damoctocog alfa pegol had mild hemophilia and one severe.
Conclusions: The real-world data obtained from the ATHNdataset confirm that some women and girls in all age groups are being treated with octocog alfa or damoctocog alfa pegol, and indicate the effectiveness of these treatments in this real-world setting. The majority have mild hemophilia A. Approximately 1/3 of the female patients were treated on some form of prophylaxis with 2/3 of these being classified as mild. Only traumatic bleeds, including traumatic joint bleeds, were noted. Three patients treated with different products before using octocog alfa had spontaneous, joint and traumatic bleeds on their previous therapies. More data are needed on the diagnosis and treatment of women with hemophilia A.
Disclosures
Charlet:Bayer U.S. LLC: Current Employment. Moulton:Bayer U.S. LLC: Current Employment. Recht:Oregon Health & Science University: Ended employment in the past 24 months; Catalyst Biosciences, CSL Behring, Genentech, Grifols, Hema Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, uniQure: Consultancy; Bayer, Biomarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, uniQure: Research Funding; Foundation for Women and Girls with Blood Disorders; Partners in Bleeding Disorders: Thrombosis and Hemostasis Societies of North America: Membership on an entity's Board of Directors or advisory committees; American Thrombosis and Hemostasis Network; Yale University School of Medicine: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal